Charith Rathnayaka, University of the Sunshine Coast; Emilie Sauret, Queensland University of Technology; Nam-Trung Nguyen, Griffith University, and Yuantong Gu, Queensland University of Technology
Carbon capture and storage (CCS) has been touted, again and again, as one of the critical technologies that could help Australia reach its climate targets, and features heavily in the federal government’s plan for net-zero emissions by 2050.
CCS is generally when emissions are captured at the source, such as from a coal-fired power station, trucked to a remote location and stored underground.
But critics say investing in CCS means betting on technology that’s not yet proven to work at scale. Indeed, technology-wise, the design of effective carbon-capturing materials, both solid and liquid, has historically been a challenging task.
So could it ever be a viable solution to the fossil fuel industry’s carbon dioxide emissions?
Emerging overseas research shows “liquid marbles” – tiny droplets coated with nanoparticles – could possibly address current challenges in materials used to capture carbon. And our modelling research, published yesterday, brings us a big step closer to making this futuristic technology a reality.
Issues with carbon capture
Under its Technology Investment Roadmap, the Morrison government considers CCS a priority low-emissions technology, and is investing A$300 million over ten years to develop it.
But the efficacy and efficiency of CCS has long been controversial due to its high-operational costs and scaling-up issues for a wider application.
An ongoing problem, more specifically, is the effectiveness of materials used to capture the CO₂, such as absorbents. One example is called “amine scrubbing”, a method used since 1930 to separate, for instance, CO₂ from natural gas and hydrogen.
The problems with amine scrubbing include its high costs, corrosion-related issues and high losses in materials and energy. Liquid marbles can overcome some of these challenges.
This technology can be almost invisible to the naked eye, with some marbles under 1 millimetre in diameter. The liquid it holds – most commonly water or alcohol – is on the scale of microlitres (a microlitre is one thousandth of a millilitre).
The marbles have an outer layer of nanoparticles that form a flexible and porous shell, preventing the liquid within from leaking out. Thanks to this armour, they can behave like flexible, stretchable and soft solids, with a liquid core.
What do marbles have to do with CCS?
Liquid marbles have many unique abilities: they can float, they roll smoothly, and they can be stacked on top of each other.
Other desirable properties include resistance to contamination, low-friction and flexible manipulation, making them appealing for applications such as gas capture, drug delivery and even as miniature bio-reactors.
In the context of CO₂ capture, their ability to selectively interact with gases, liquids and solids is most crucial. A key advantage of using liquid marbles is their size and shape, because thousands of spherical particles only millimetres in size can directly be installed in large reactors.
Gas from the reactor hits the marbles, where it clings to the nanoparticle outer shell (in a process called “adsorption”). The gas then reacts with the liquid within, separating the CO₂ and capturing it inside the marble. Later, we can take out this CO₂ and store it underground, and then recycle the liquid for future processing.
This process can be a more time and cost-efficient way of capturing CO₂ due to, for example, the liquid (and potentially solid) recycling, as well as the marbles’ high mechanical strength, reactivity, sorption rates and long-term stability.
So what’s stopping us?
Despite recent progress, many properties of liquid marbles remain elusive. What’s more, the only way to test liquid marbles is currently through physical experiments conducted in a laboratory.
Physical experiments have their limitations, such as the difficulty to measure the surface tension and surface area, which are important indicators of the marble’s reactivity and stability.
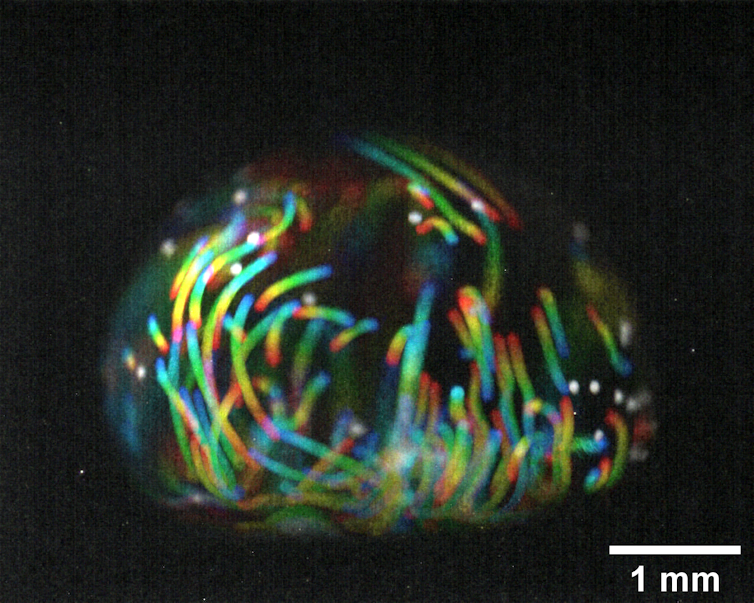
In this context, our new computational modelling can improve our understanding of these properties, and can help overcome the use of costly and time-intensive experiment-only procedures.
Another challenge is developing practical, rigorous and large-scale approaches to manipulate liquid marble arrays within the reactor. Further computational modelling we’re currently working on will aim to analyse the three-dimensional changes in the shapes and dynamics of liquid marbles, with better convenience and accuracy.
This will open up new horizons for a myriad of engineering applications, including CO₂ capture.
Beyond carbon capture
Research on liquid marbles started off as just an inquisitive topic around 20 years ago and, since then, ongoing research has made it a sought-after platform with applications beyond carbon capture.
This cutting-edge technology could not only change how we solve climate problems, but environmental and medical problems, too.
Magnetic liquid marbles, for example, have demonstrated their potential in biomedical procedures, such as drug delivery, due to their ability to be opened and closed using magnets outside the body. Other applications of liquid marbles include gas sensing, acidity sensing and pollution detection.
With more modelling and experiments, the next logical step would be to scale up this technology for mainstream use.
Charith Rathnayaka, Lecturer in Mechanical Engineering, University of the Sunshine Coast; Emilie Sauret, Professor, Queensland University of Technology; Nam-Trung Nguyen, Professor and Director of Queensland Micro- and Nanotechnology Centre, Griffith University, and Yuantong Gu, Professor, Mechanical Systems and Asset Management, Queensland University of Technology
This article is republished from The Conversation under a Creative Commons license. Read the original article.